The chemical structure of carboxylic acid derivatives affects the relative chemical reactivity of the common acid derivatives.
Let’s view the carboxylic acid derivatives as an acyl group, R-C=O, attached to a substituent (X). The order of reactivity in nucleophilic addition-elimination reactions for acid derivatives is: acid anhydrides > esters > amides. The reactivity of acid derivatives in nucleophilic addition-elimination reactions decreases with increasing basicity of the leaving group.
These derivatives also undergo a nucleophilic substitution reaction with a nucleophile (Nu) as shown below. The reactivity of these derivatives towards nucleophilic substitution is governed by the nature of the substituent X present in the acid derivative and plays a part in electronic effects.

If the substituent (X) is electron donating, it reduces the electrophilic nature of the carbonyl group by neutralizing the partial positive charge developed on the carbonyl carbon, and thus makes the derivative less reactive to nucleophilic substitution.
If the substituent (X) is electron withdrawing, then it increases the electrophilic nature of carbonyl group by pulling the electron density of the carbonyl bond towards itself, making the carbonyl carbon more reactive to nucleophilic substitution.
The size of the substituents (steric effect) also matter in dictating whether or not a reaction with proceed. This is called steric hindrance. In the context of carboxylic acid derivatives, the size of the leaving group can affect the ability of a nucleophile to access the carbonyl carbon, thus affecting the reactivity of the derivative to nucleophilic substitution.
Lastly, the strain a compound is under can affect its reactivity. A good example would be β-lactams, commonly used as an antibiotic. In the example of penicillin (seen below), a commonly used antibiotic, the strain of being in two rings causes it to be very easily hydrolyzed.
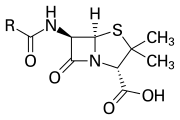

Key Points
• The nature of the substituent will dictate electronic effects.
• The size of the substituent will dictate steric effects.
• If a compound is under more strain, it will be more reactive.
Key Terms
Steric hindrance: the size of the substituent affecting a reaction’s ability to proceed
Nucleophilic addition-elimination: A two-stage reaction process of an addition reaction followed by an elimination reaction. This gives an overall effect of substitution, and is the mechanism of the common nucleophilic acyl substitution often seen with esters, amides, and related structures.
Electronic effect: Influences the structure, reactivity, or properties of molecule but is neither a traditional bond nor a steric effect. The term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry (stereochemistry) of a molecule.
Steric effects: Nonbonding interactions that influence the shape (conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which usually dictate shape and reactivity. Steric effects result from repulsive forces between overlapping electron clouds.
Steric hindrance: The prevention or retardation of inter- or intramolecular interactions as a result of the spatial structure of a molecule.
Strain: An increase in molecular potential energy due to electron repulsion or a deviation from ideal geometry.