DNA is a nucleic acid that contains monomer nucleotide units formed into a polymer and is held in a double helix structure by hydrogen bonding between nitrogenous bases of the two antiparallel strands.
DNA contains a large range of important chemical concepts. As a polymer, DNA is made up of nucleotide monomers which each contain a nitrogenous base, ribose sugar, and phosphate group. The nitrogenous bases of one strand in the double-stranded structure form hydrogen bonds to the other strand to form a double helix. These hydrogen bonds form between the amino groups on one base and the amino or carbonyl groups on the adjacent nitrogenous base. These bases match up in complementary pairs due to the number of hydrogen bonds and relative sizes of the bases. Guanine binds to Cytosine via 3 hydrogen bonds, as shown below, whereas Adenine binds to Thymine via 2 hydrogen bonds, .

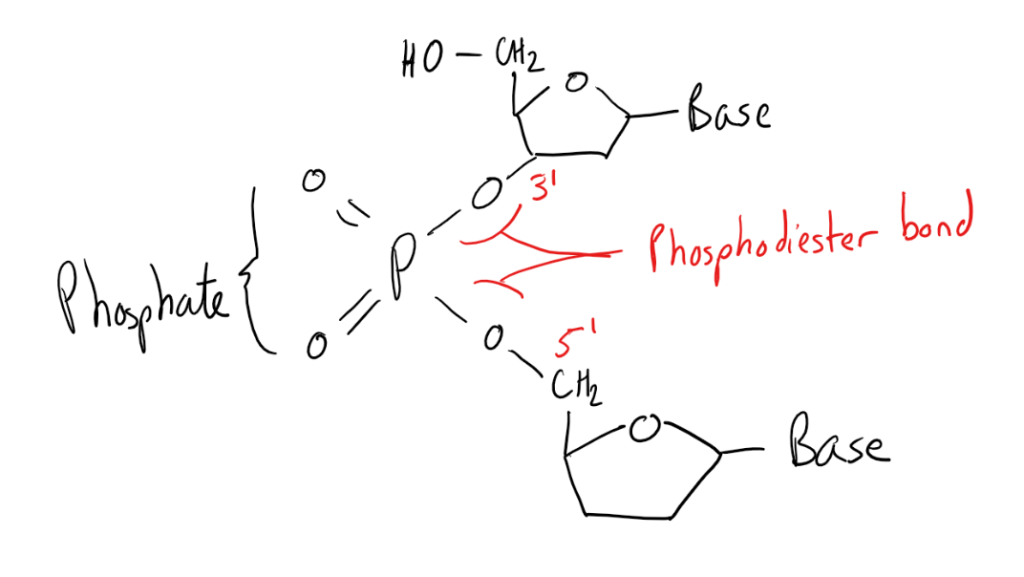
The chemistry of the phosphate groups forms another important part of the DNA structure, joining together the ribose sugars and bases to form a polymer with phosphodiester bonds. The phosphate groups form bonds between the 3rd carbon (3’) of the ribose sugar of one base and the 5th carbon (5’) of the ribose sugars of the other base, forming the phosphate backbone of a DNA strand.
Practice Questions
Khan Academy
Applications of Hard-Soft Acid-Base theory
MCAT Official Prep (AAMC)
Section Bank C/P Section Question 81
Practice Exam 2 C/P Section Question 26
Practice Exam 1 B/B Section Question 46
Key Points
• DNA nitrogenous bases are important in bonding two strands together to form the double helix using weak intermolecular hydrogen bonding forces.
• The backbone of DNA is made up of phosphate molecules that form bonds between ribose sugars (through condensation reactions) to link monomers together to form a long polymer strand.
Key Terms
DNA: Deoxyribonucleic acid, the genetic material found within the cell nucleus.
hydrogen bond: a weak intermolecular attraction between a hydrogen atom and an electronegative element such as oxygen, nitrogen or fluorine
amino group: a chemical functional group which contains a nitrogen bonded to hydrogen atoms
carbonyl group: a chemical functional group characterized by a carbon double bonded to an oxygen atom
complimentary base-pairing: the pairing of opposite bases on opposite strands of a DNA double helix, A pairs to T and G pairs to C
phosphodiester bond: linkage that occurs when two hydroxyl groups in a phosphate molecule react with hydroxyl groups on ribose sugars to form two ester bonds